| DESCRIPTION |
|
Test method |
| Appearance |
White or nearly white particles |
visual |
| Solubility |
Practically insoluble in water, freely soluble in acetone, in methanol and in methylene chloride; it dissolves in dilute solutions of alkali hydroxides and carbonates |
informative |
| Physical Parameters |
|
|
| Particle size distribution |
≥ 75 % 200 – 500 μm |
Ph. Eur. |
| Bulk density [g/ml] |
0.5 – 0.7 (informative) |
Ph. Eur. |
| IDENTIFICATION |
|
|
| A Melting point [°C] |
75 – 78 |
Ph. Eur. |
| C Infrared Absorption |
IR-spectrum equivalent reference standard |
Ph. Eur. |
| CHEMICAL PARAMETERS |
|
|
| Appearance of solution |
Clear and colorless |
Ph. Eur. |
| Optical rotation [°] |
– 0.05 to + 0.05 |
Ph. Eur. |
| Related Substances |
Specified impurities* |
J, A, N ≤ 0.15 % |
Ph. Eur. |
| |
Other detectable impurities |
B, D, E, K, L, M ≤ 0.05 % |
Ph. Eur. |
| |
Unspecified impurities |
≤ 0.05 % for all unidentified peaks |
Ph. Eur. |
| |
Total impurities |
≤ 0.2 % (Peaks < 0.03 % are disregarded) |
Ph. Eur. |
| Elemental Impurities [ppm] |
Fe ≤ 1300, Cr ≤ 25 |
Ph. Eur. |
| Loss on drying [%] |
≤ 0.5 |
Ph. Eur. |
| Sulphated ash [%] |
≤ 0.1 |
Ph. Eur. |
| Assay [%] |
98.5 to 101.0 |
Ph. Eur. |
| Residual solvents [ppm] |
Acetone |
≤ 100 |
Ph. Eur. |
| Hexane |
≤ 290 |
| Isopropyl alcohol (IPA) |
≤ 250 |
| Trichloroethylene |
≤ 80 |
| Methanol** |
≤ 500 |
| MICROBIOLOGICAL QUALITY |
|
|
| TAMC [CFU/g] |
≤ 103 |
Ph. Eur. |
| TYMC [CFU/g] |
≤ 102 |
Ph. Eur. |
| E.coli |
absence in 1 g sample |
Ph. Eur. |
* The starting material Ibuprofen Ph. Eur. for Ibuprofen DC100 does not contain the impurity F.
** Not used in the process, test included for compliance with certificate of suitability.
Organic solvents in accordance to Ph. Eur., 5.4 and USP <467> (CPMP/ICH/283/95) are not used neither by manufacturing of Ibuprofen DC100 nor by cleaning of equipment.
The starting material of Ibuprofen DC100 is exclusively of synthetic origin. A contamination with animal material by manufacturing, storage or shipment in the original closed containers will not occur. Therefore, Ibuprofen DC100 is free from Bovine Spongiform Encephalopathy (BSE) and Transmissible Spongiform Encephalopathy (TSE). Ibuprofen DC100 complies with the requirements of EMA/410/01 rev.3.




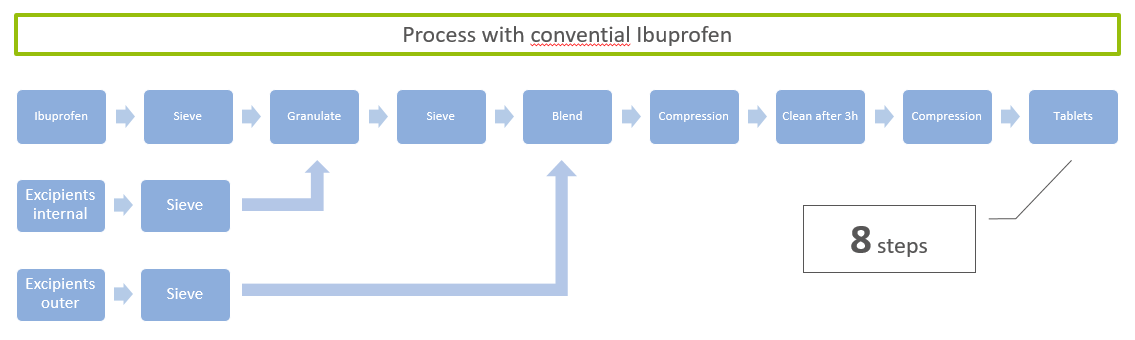

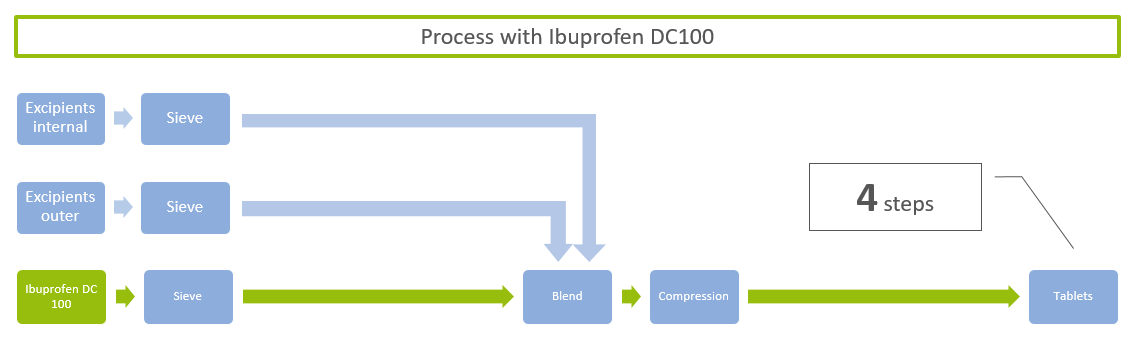


 ingredientpharm
ingredientpharm ingredientpharm
ingredientpharm ingredientpharm
ingredientpharm ingredientpharm
ingredientpharm
 ingredientpharm
ingredientpharm ingredientpharm
ingredientpharm ingredientpharm
ingredientpharm ingredientpharm
ingredientpharm ingredientpharm
ingredientpharm ingredientpharm
ingredientpharm ingredientpharm
ingredientpharm ingredientpharm
ingredientpharm