Tableting of granulated Ibuprofen
Tableting of granulated Ibuprofen avoids many issues which appear when using conventional drug. Without a doubt, the comparable low melting point of Ibuprofen leads to a sticky behavior during compression processes. Hence, this might result in a quality issue or decrease in customers’ compliance. In the following, a study of tableting granulated and conventional Ibuprofen compares the differences of both physical forms. Not only the tablet dimension and mass, but also the dissolution kinetics are investigated.
Find the right dosage
Speaking about consumer products such as tablets, diverse Ibuprofen concentrations and thus dosages are requested. The smallest dosage represents 200 mg of the drug, while high-dose formulations contain up to 800 mg Ibuprofen. Assuming a drug load of 80%, the respective tablet mass can be evaluated. Table 1 shows exemplary drug formulations. Correspondingly, an assumed drug load of 80% and a targeted dosage of 800 mg results in a tablet mass of 1000 mg.
| Dosage [mg] | Product | Tablet mass [mg] | API load [%] |
| 200 | Ibuprofen DC 100 tablet acute | 250 | 80 |
| 400 | Ibuprofen DC 100 tablet acute | 500 | 80 |
| 400 | Ibuprofen DC 100 tablet | 500 | 80 |
| 600 | Ibuprofen DC 100 tablet | 750 | 80 |
| 800 | Ibuprofen DC 100 tablet | 1000 | 80 |
Table 1: Dosage variations of Ibuprofen formulations as tablets with a drug load of 80%. Dosage and tablet mass are given in [mg], drug load in [%], respectively.
Expert’s opinion
Go for Ibuprofen DC100 when working on modified/sustained release formulations with a drug load of more than 90%. The granulated drug form contains 100% Ibuprofen. It allows easier coating and compression processes. The tablet size shrinks due to the high accessible drug load.
Modified release and high drug load – how to combine
In the following, we focus on modified release matrix tablets with a drug load of more than 90%. There are two main attempts behind these goals. On the one hand, the sustained release profile shall enable a continuous drug release with time. On the other hand, the high drug load shall decrease the tablet size and thus increase the customers’ compliance.
Obviously, a dosage strength at 800 mg and a sustained release between 12 to 24 hours are desired beside the smallest possible tablet size.
In this study, a market product made of conventional Ibuprofen (drug load: 66%) is compared with a conceptual formulation working on Ibuprofen DC100 with a drug load of 90%. Both formulations are expressed as tablets and have a drug dosage at 800 mg. The market product has an overall tablet mass of 1220 mg while the Ibuprofen DC100 tablet has a weight of 833 mg. It is clear, that the tablet of the market product has generally larger dimensions. Table 2 compares the physical properties of the tablets. Figure 1 shows a direct comparison of both tablets.
| Ibuprofen DC100 | Market product | |
| Mass [mg] | 833 | 1220 |
| Drug dosage [mg] | 800 | 800 |
| Tablet dimension [mm] | 18 x 8 | 18.7 x 8.2 |
| Tablet height [mm] | 7.15 | 8.85 |
Table 2: Comparison of tablets based on Ibuprofen DC100 and conventional drug (market product). Mass and dosage are given in [mg], tablet dimension and height are expressed in [mm].

Figure 1: Tableting of granulated Ibuprofen formulations: tablet sizes. Left: Ibuprofen DC100; right: market product. Dimensions are given in Table 2.
Dissolution profiles
Consequently, when it comes to dissolution of Ibuprofen, both physical forms generally coincide. Anyhow, the granular form of Ibuprofen DC100 allows easier coating with further layers of excipients for modified release characteristics. A main reason might be the smoother tablet surface after the compression process. In turn, this enables the formulation scientist to precisely define the dissolution profile. In this study, a sustained drug release was desired and is shown in Figure 2. Figure 2 presents the dissolution of the market product and the Ibuprofen DC100 tablet. While the market product reaches a level of almost full release after 480 minutes, the Ibuprofen DC100 tablet reaches this level after 1300 minutes. Additionally, the sustained release over this extended time seems gratefully linear.
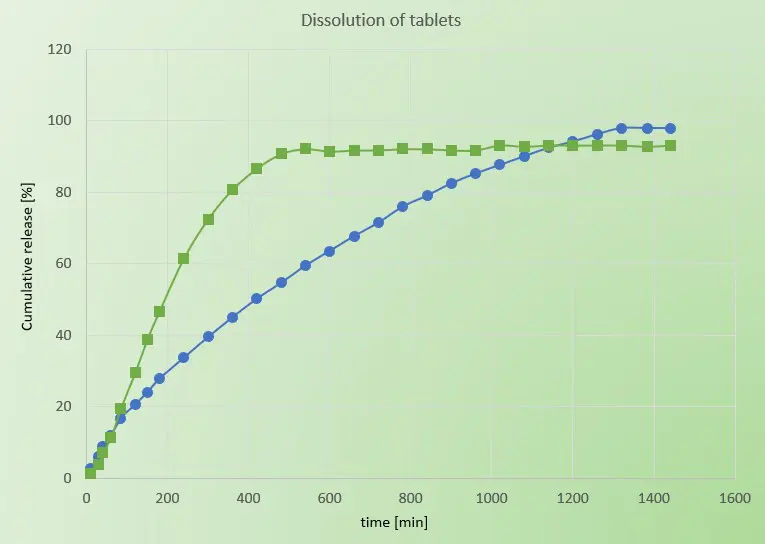
Figure 2: Dissolution curve of both tablets. Cumulative release as a function of time for the market product (green) and Ibuprofen DC100 tablet (blue).
Conclusion
Tableting of granulated Ibuprofen DC100 is meaningful, as it allows designing a precise dissolution of the drug. In a direct comparison with a market product with equal amount of drug dosage, the Ibuprofen DC100 tablet remains smaller in any dimension, while allowing a sustained drug release with an almost linear drug release over a time frame of 24 hours.

 ingredientpharm
ingredientpharm