Ibuprofen DC100 – a grade for direct compression
Ibuprofen as a popular drug
Before speaking on the Ibuprofen DC100 grade for direct compression, we first analyze why improvements on Ibuprofen formulations are advisable. Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) for the treatment of pain (popular disease: headache), fever or high temperature, and inflammation. The drug is used in diverse dosage forms, generally the uptake is favored intravenously or orally. In fact, the oral uptake allows several dosage forms, such as sachets, stick packs or tablets. These formulations might have designed drug dissolution profiles ranging from immediate release, modified release to controlled release.
The main issue with Ibuprofen
There are some issues with the drug in oral dosage forms. In case of sachets or stick packs, taste masking is a crucial factor for high customer compliance, while in the case of tablets, the manufacturing of Ibuprofen is somewhat tricky: the drug is hard to process. The low compression and compaction properties are the main obstacle in processing the formulation.
Compression of Ibuprofen demands sophisticated treatment of the drug to achieve compressibility. Long compression cycles are critical as the product tends to stick after a while due to the low melting point and especially with using low compression forces leads into problems. In further report we will shed some light on rough or even defective tablet surfaces.
Expert’s opinion
Ibuprofen DC100 (Ph. Eur.) can simplify the production process of your oral Ibuprofen formulation with great advances. Use the improved physical form of Ibuprofen in your designed formulation for increasing the process simplicity or turning the efficiency of coating and layering properties.
Solution: Ibuprofen for direct compression
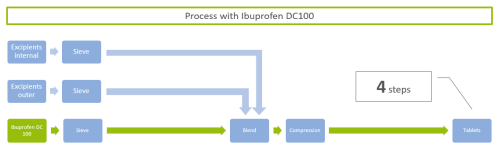
Ibuprofen for direct compression, namely Ibuprofen DC100, particles are granulated by patented process technologies and consists of 100% pure active without additional excipients. This allows an easy substitution in Ibuprofen formulations while keeping the registered composition in the outer phase. Furthermore, process steps in tableting are reduced: The process with conventional Ibuprofen includes in this exemplary case 8 steps including sieving, granulating with internal and outer excipients, blending, compression, cleaning, and so on (Figure 1).
Figure 1: Exemplary process with conventional Ibuprofen: 8 steps required.
Driving the process with the granulated Ibuprofen DC100, the process steps reduce by 50% and result in 4 steps, such as sieving, blending, compression (Figure 2). The main process steps include the mixing with internal and outer excipients which cannot be avoided.
Figure 2: Exemplary process with granulated Ibuprofen DC100: 4 steps remaining – Ibuprofen for direct compression.
Simplifying complex formulations
Granulated Ibuprofen DC100 has a granule size in the range between approximately 200 µm and 500 µm with a bulk density at 0.5 g/ml to 0.7 g/ml. It satisfies regulations following Ph. Eur. Speaking about a drug concentration at 100%, the granulated Ibuprofen easily substitutes conventional Ibuprofen in respective formulations. Thereby, the pharmaceutical formulations might provide immediate or extended drug release profiles. Thus, Ibuprofen DC100 can replace current Ibuprofen grade with only minor regulatory changes since no other excipient is employed. Ibuprofen DC100 for direct compression is not only useful for tablets, but also for sachets or stick pack applications: Taste-masking with these granules is comparable simple realize and results in an increased client compliance.
The high drug concentration provides maximum freedom in formulation of dosage forms e.g., tablet size and release characteristics to achieve better patients’ compliance.
Find your solution
See how Ibuprofen DC100 can simplify your production process. Study the technical information or request a sample for your lab testing.



 ingredientpharm
ingredientpharm ingredientpharm
ingredientpharm